

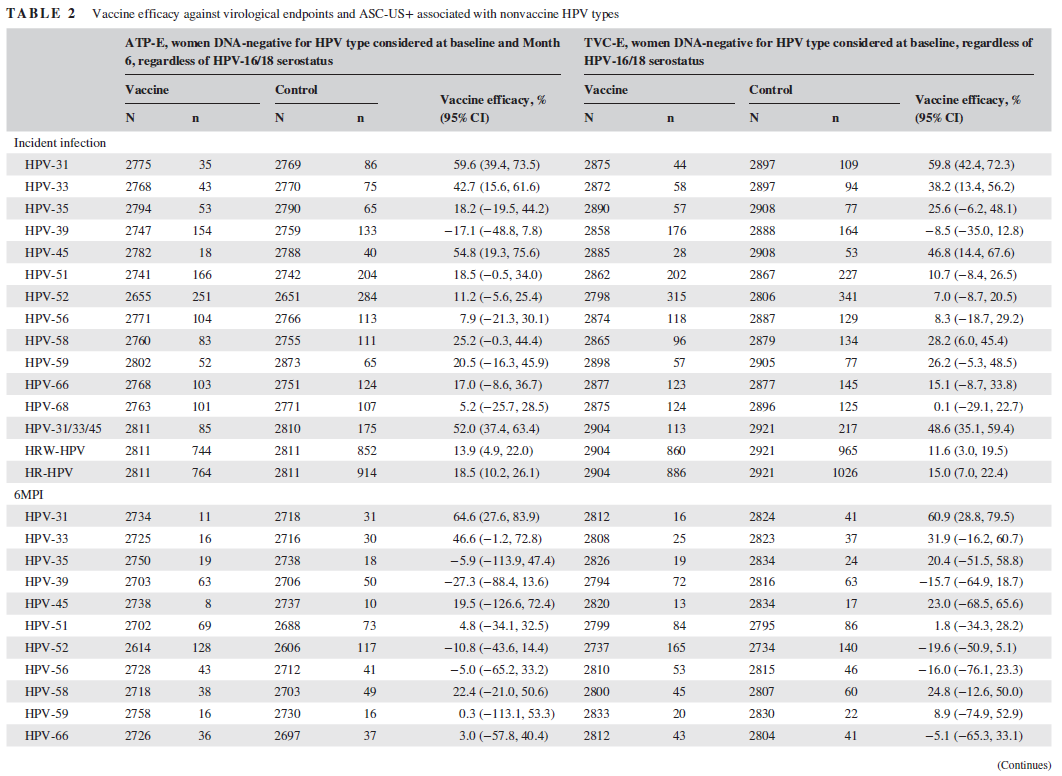
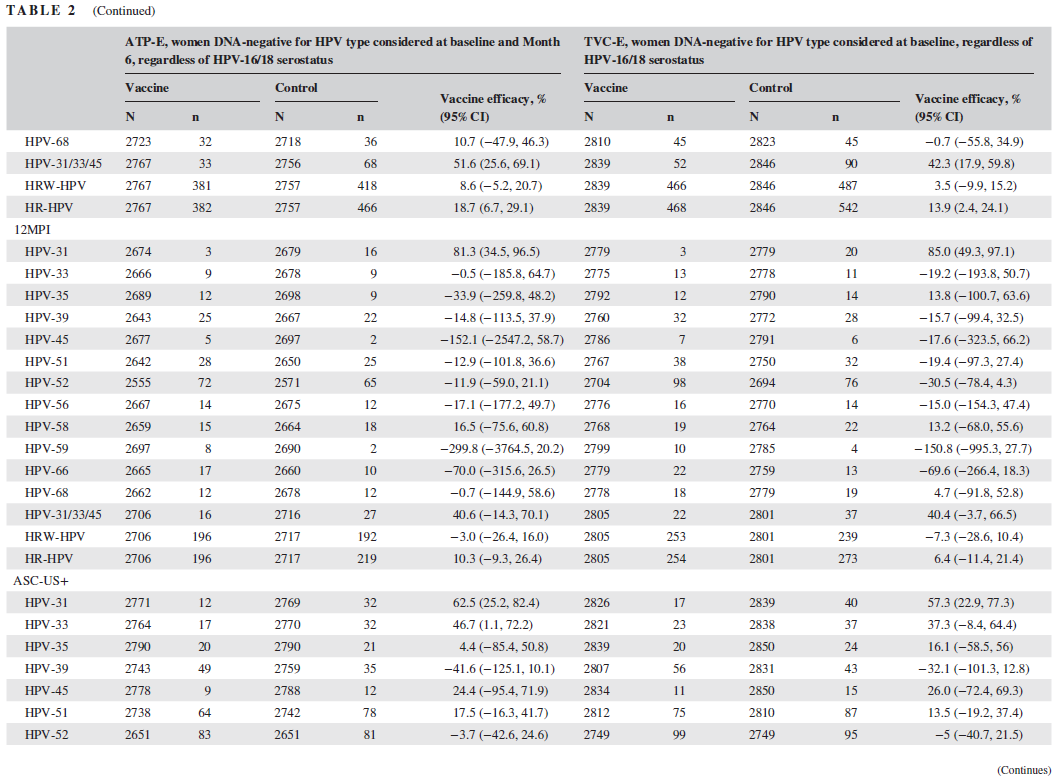
該結果基于中國婦女(nǚ)18-25歲群體AS04‐HPV‐16/18疫苗效力、安全性和(hé)免疫原性的(de)随機對(duì)照(zhào)雙盲實驗。HPV2價疫苗對(duì)HPV‐31 (59.6% [95% CI: 39.4, 73.5])、HPV‐33 (42.7% [95% CI: 15.6, 61.6])和(hé)HPV‐45 (54.8% [95% CI: 19.3, 75.6])的(de)機會感染具有保護效力,對(duì)6個(gè)月(yuè)和(hé)12個(gè)月(yuè)持續HPV‐31感染具有保護效力 (分(fēn)别爲64.6% [95% CI: 27.6, 83.9]和(hé)81.3% [95% CI: 34.5, 96.5])。此外對(duì)HPV‐31 (62.5% [95% CI: 25.2, 82.4])和(hé)HPV‐33 (46.7% [95% CI: 1.1, 72.2])相關的(de)ASCUS+具有保護效力。對(duì)HPV‐31/33/45相關的(de)CIN1+的(de)疫苗效力爲36.1% (95% CI: −80.5, 79.0),對(duì)HPV‐31/33/45相關的(de)CIN2+的(de)疫苗效力爲74.9% (95% CI: −25.8, 97.4)。

參考文獻:Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, Pan QJ, Zhang WH, Zhao FH, Zhang CF, Yang X, Yu JX, Zhu J, Zhu Y, Chen F, Zhang Q, Wang H, Wang C, Bi J, Xue S, Shen L, Zhang YS, He Y, Tang H, Karkada N, Suryakiran P, Bi D, Struyf F. Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18-25 years: End-of-study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019 Oct;8(14):6195-6211. doi: 10.1002/cam4.2399. Epub 2019 Jul 15. PMID: 31305011; PMCID: PMC6797633.
安徽省腫瘤防治所 安徽省腫瘤防治辦公室
Copyright © 2019 www.ahzlfzs.com,All rights reserved
版權所有 © 安徽腫瘤防治所 未經許可(kě) 嚴禁複制 備案号:
地址:合肥市蜀山區(qū)績溪路218号 電話(huà):0551-62922302